Learning Fear Extinction is Driven by Dopamine Neurons in the Midbrain
Post by Rebecca Hill
The takeaway
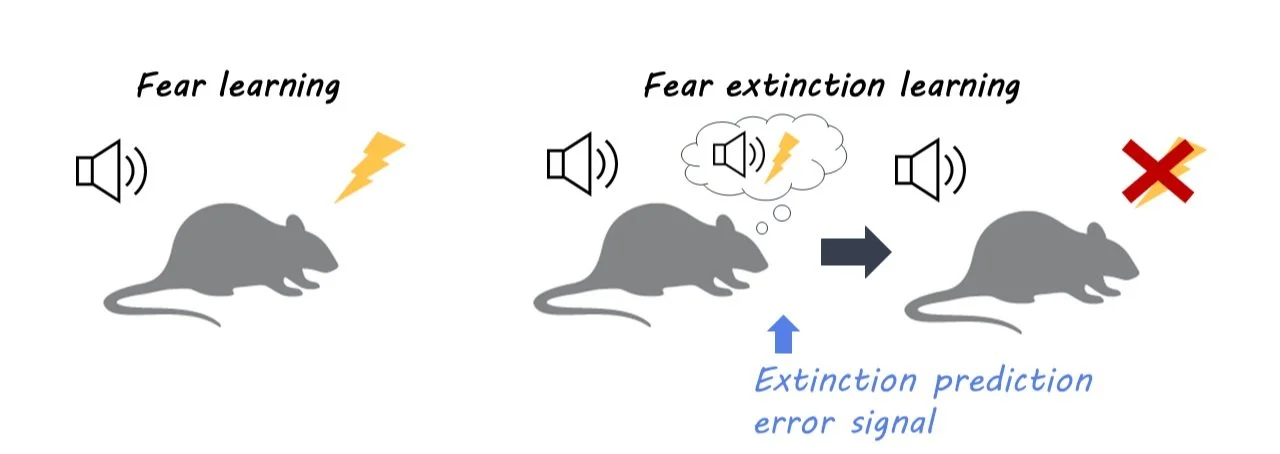
Dopamine neurons help signal fear extinction - the ability to stop fear responses to stimuli that are no longer dangerous. Neuronal pathways in the midbrain are required for dopamine signaling in fear extinction learning.
What's the science?
In order to adapt to signals that are no longer dangerous, animals must learn how to stop fear responses through fear extinction learning. Individuals with post-traumatic stress disorder (PTSD) also require this ability to inhibit fear responses. While previous research has shown the involvement of dopamine neurons in learning fear extinction, it is still unclear which specific neuronal pathways are involved. This week in Neuron, Salinas-Hernandez and colleagues identified these dopamine pathways in the midbrain by measuring dopamine neuron activity.
How did they do it?
In order to learn to associate signals with new and different outcomes (dangerous or not dangerous), animals must use prediction errors: where a prediction is met with a different result, an error. To capture brain activity associated with these prediction errors, the authors used fiber photometry to measure activity-dependent calcium signals from dopamine neurons while mice underwent fear extinction paradigms. This involved first playing mice a tone to habituate them to the signal. Next, the mice are conditioned to fear the tone, by pairing it with a foot shock. Last, they played the tone by itself for two days to create the extinction of the fear response. The authors measured dopamine neuron activity in the midbrain of mice both before the fear conditioning and after. They also measured the response to positive signals by rewarding mice with sugar water for nose-poking a target area. To observe whether the fear extinction response was dopamine neuron dependent, the authors optogenetically inhibited dopamine neurons in NAc. Finally, the authors used chemogenetic inhibition to test whether neurons in specific areas of the midbrain were used to signal for prediction error signals.
What did they find?
The authors found that dopamine neurons showed more activity in certain areas of the midbrain — the ventral tegmental area (VTA) and the nucleus accumbens (NAc) — during extinction than during habituation. This suggests these pathways of dopamine learning are used in fear extinction learning. Specifically, neurons in the anteromedial NAc responded with more activity than in other areas. This suggests the prediction error signal is carried to this specific area of the NAc. The authors also saw more calcium signaling, and higher activity, when mice were rewarded by sugar water, which suggests these areas also are activated by rewards. Finally, when dopamine neurons were inhibited in NAc, mice were less able to exhibit extinction learning and continued to display a fear response to just the tone. This provides further evidence that dopaminergic neurons projecting to NAc control fear extinction. Finally, when the authors inhibited dopamine neurons that projected to the VTA (a region with neurons that project to the NAc), mice could not learn fear extinction, suggesting that VTA-projecting dopamine neurons are required for prediction error generation.
What's the impact?
This study is the first to show that midbrain regions control the fear extinction learning for dopamine neurons. This work furthers our understanding of brain regions involved in fear extinction learning and could have future implications in humans. More broadly, it could point to possible treatments for disorders such as PTSD.