How Do Pathogens Invade the Brain?
Post by Anastasia Sares
What's the science?
Infections of the central nervous system can be life-threatening. Their dynamics are changing, influenced by globalization, climate change, and other factors. Meningitis alone affects 1.2 million people, and certain subtypes can be extremely deadly. This week in Neuron, Cain and colleagues reviewed the multiple ways that pathogens (bacteria, fungi, viruses, toxins, and parasites) can make their way into the brain.
What have we learned?
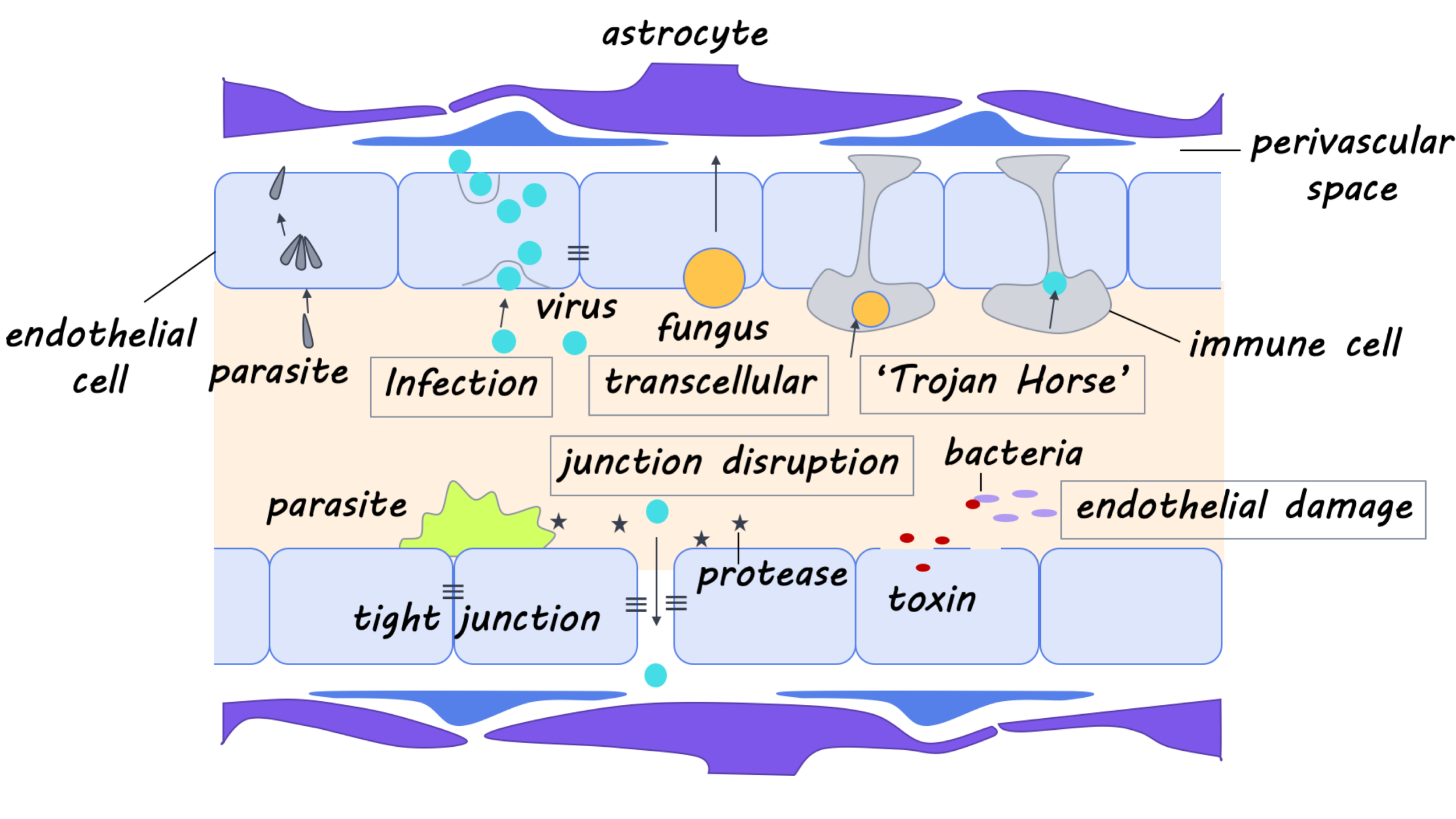
Our body has many lines of defense against would-be invaders, and the brain is one of the most protected parts of the body. First, there are the skin and the skull, which provide formidable physical barriers to entry. Below the skull is a leathery layer called the dura mater. Below this is the spongy arachnoid mater, housing a number of blood vessels. Finally, there is the pia mater, a thin and delicate layer that is almost like shrink-wrap around the brain. Unlike in the rest of the body, blood nutrients do not migrate freely into brain tissue. Blood vessels are tightly sealed, forming what we call the “blood-brain-barrier.” The cells surrounding the blood vessels get to pick and choose what nutrients they want and transport them selectively across their membranes. Even some of our own immune cells are not allowed past this barrier. Instead, the brain and spinal cord have a separate, dedicated system for pumping nutrients around, the cerebrospinal fluid (CSF), and in-house immune cells. At this point, you might think the brain is extremely well protected, however, there are surprisingly still ways for invaders to penetrate these defenses.
So, how do some pathogens still manage to get inside the brain? Some only need to get through one or two layers of the brain’s protection to be dangerous. Meningitis is a swelling of the membranes that protect the brain: it typically happens when a pathogen gets in between those layers and multiplies there. Even bacteria that commonly exist in some people’s noses and throats can be life-threatening if they make their way into these spaces. Another point of entry is through the peripheral nerves. If a pathogen manages to get inside neurons somewhere else in the body, it can make its way back along the nerves up to the spinal cord and brain. This is called retrograde transport and allows the virus to bypass the blood-brain barrier completely (used by rabies, poliovirus, and herpes viruses including chickenpox).
Other pathogens confront the blood-brain barrier head-on. This means dealing with the endothelial cells surrounding blood vessels, which can be thought of as “gatekeeper cells,” tightly restricting access to brain tissue. One way to overcome this is to hijack the mechanisms usually involved in nutrient transport (one of the many strategies of West Nile Virus). Then, there is the “Trojan Horse” method: hitch a ride inside the few immune cells that are allowed to squeeze through the blood-brain barrier (another strategy for West Nile and many other viruses). Still, other pathogens specifically infect the gatekeeper cells themselves (Toxoplasma gondii in mice). If an infected cell ruptures, the pathogens can then cross into the brain itself. Finally, invaders can also disrupt the function of gatekeepers or other cells that help maintain the blood-brain barrier so that it gradually degrades. Or, they can cause an inflammatory response in the brain, which has a similar effect (used by S. pneuomniae).
What else is new?
New technologies have allowed us to better understand the different ways that pathogens enter the central nervous system. For example, we are able to grow and compartmentalize individual neurons in different chambers and see their networks more clearly; we can also image live, fluorescent viruses as they move. The ability to sequence and modify genomes has allowed us to determine which genes and proteins are used by these pathogens and what happens when we turn them on or off. All of these advances have allowed us to understand things like how viruses can move up nerves, co-opting cellular transport machinery along the way.
What’s the bottom line?
The more we know about how pathogens overcome our body’s natural defenses, the better we can combat them with medicine. With some work, we might also be able to repurpose the mechanisms used by neuro-invasive pathogens to deliver life-saving treatments past the blood-brain barrier.
Cain et al. Mechanisms of Pathogen Invasion into the Central Nervous System. Neuron (2019).Access the original scientific publication here.