Reducing Neuroinflammation with Brain-Specific Gene Delivery
Post by Leanna Kalinowski
The takeaway
Acute central nervous system (CNS) trauma, which is present in neurological disorders such as multiple sclerosis, triggers the activation of immune cells in the brain causing inflammation. Researchers have developed a brain-specific gene delivery system for brain interleukin-2, which is effective in preventing and treating these neurological disorders in mouse models.
What's the science?
Acute central nervous system (CNS) trauma is caused by diverse neurological injuries and illnesses, like traumatic brain injury (TBI), multiple sclerosis, or stroke. CNS trauma leads to the damage and loss of neurons, which can result in changes in cognition, sensorimotor function, and personality. Acute CNS trauma also triggers the activation of immune cells in the brain – like microglia and astrocytes – which leads to further negative effects.
Brain interleukin-2 (IL-2) has been identified as a potential treatment option for acute CNS trauma. IL-2 works by supporting the survival and proliferation of regulatory T (Treg) cells, which have been shown to inhibit neuroinflammation. However, while IL-2 treatment shows promise, it is unclear whether such treatment can be localized to the brain rather than inducing unintended immune effects elsewhere in the body. This week in Nature Immunology, Yshii and colleagues (1) developed a new delivery system for brain-specific IL-2 treatment and (2) tested its effectiveness in preventing and treating models of acute CNS trauma.
How did they do it?
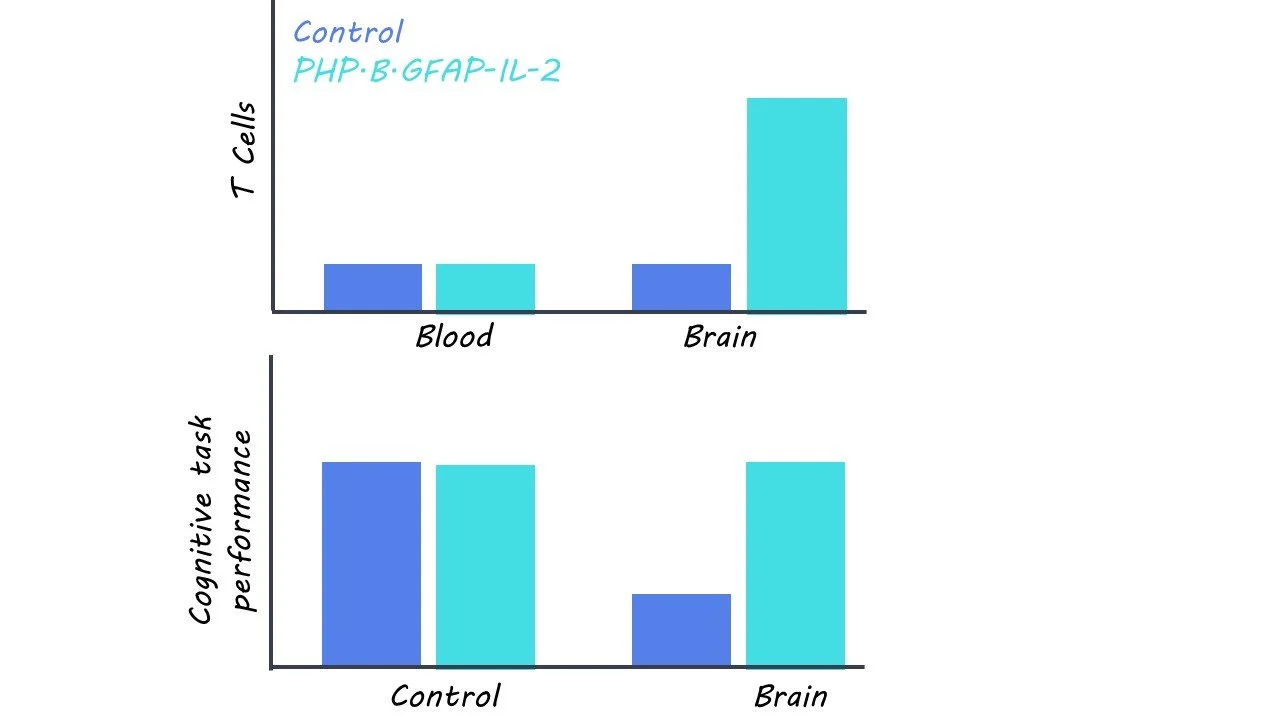
First, the researchers developed a gene delivery approach to increase IL-2 expression in astrocytes, which are a type of glial cell that responds to brain injury. To ensure that they specifically target astrocytes in the brain, the researchers first developed a vehicle for gene delivery by combining (1) a PHP.B-enhanced adeno-associated virus (AAV-PHP.B), which can be injected intravenously and delivers substances across the blood-brain barrier and (2) a modified GFAP promoter, which restricts gene expression to just astrocytes. Then, they incorporated the gene for IL-2 (PHP.B.GFAP-IL-2) into this virus, so that IL-2 is made by astrocytes in the brain, with subsequent measures of IL-2 and Treg cell production in the brain.
Then, they tested the effectiveness of PHP.B.GFAP-IL-2 in preventing acute CNS trauma in mouse models. Mice were first treated either with PHP.B.GFAP-IL-2 or a control virus (PHP.B) and then underwent a procedure to induce TBI. Fourteen days after TBI, neurological damage was assessed using MRI and histology, and behavior was assessed by running mice through two memory tests – Morris Water Maze and the Novel Object Recognition test. To examine other common sources of acute CNS trauma, this procedure was repeated for two other pathologies – ischemic stroke and multiple sclerosis.
Finally, to test the effectiveness of PHP.B.GFAP-IL-2 in curative mouse models of acute CNS trauma, they first subjected mice to acute CNS trauma (i.e., TBI, stroke, and multiple sclerosis). Then, they administered either PHP.B.GFAP-IL-2 or a control virus (PHP.B) and ran the same test battery as above.
What did they find?
First, the researchers found that PHP.B.GFAP-IL-2 was successful in delivering the IL-2 gene to the brain with expression in astrocytes. Over the course of fourteen days following administration, there was a threefold increase in IL-2 production. This was accompanied by an increase in Treg cell production, which was not observed outside of the brain and was not accompanied by any adverse symptoms.
Then, they found that pre-treatment of PHP.B.GFAP-IL-2 decreased the loss of cortical tissue and behavioral deficits in mice with TBI relative to those that received a control virus. Similar protective effects were observed in mouse models of ischemic stroke and multiple sclerosis. They also found that mice treated with PHP.B.GFAP-IL-2 after TBI also experienced lower levels of brain damage than control mice. This effect was mirrored in multiple sclerosis mice, but not in ischemic stroke mice, suggesting that the damaging effects of stroke are too rapid for this treatment to be effective before irreversible brain damage sets in.
What's the impact?
Taken together, the results from this study provide evidence for the effectiveness of brain-specific IL-2 gene delivery in preventing and treating acute CNS trauma. Future work is needed to determine whether such treatment is effective in humans with neurological injuries and diseases. Further, this study may also pave the way for the development of gene delivery therapies for other neurological disorders that require brain-specific delivery.