REM Sleep is Necessary for Experience-Dependent Plasticity
Post by Elisa Guma
What's the science?
Sleep is an important physiological process. Many lines of evidence suggest that sleep is important for regulating neuronal plasticity during brain development and after learning. A prominent feature of experience-dependent synaptic plasticity is the pruning of existing synapses during development and adulthood, which is thought to contribute to changes in neuronal activity. This week in Nature Communications, Zhou and colleagues investigated the role of sleep in dendritic spine elimination in the developing visual cortex and frontal association cortex, respectively, to try to unravel the role of sleep in experience-dependent synapse elimination.
How did they do it?
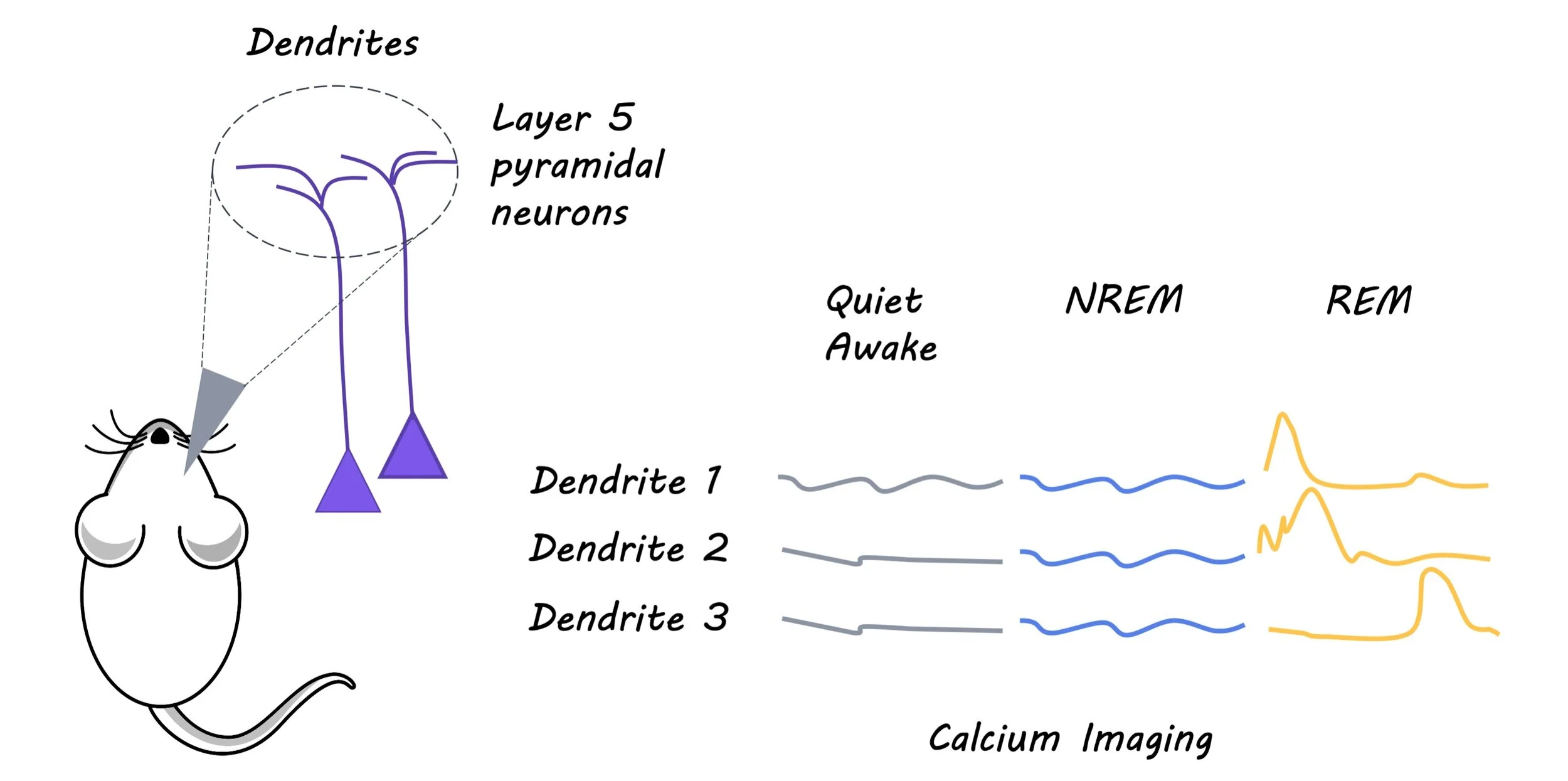
To evaluate the effect of sleep and sleep deprivation on experience-dependent spine elimination the authors used transgenic mice expressing a yellow fluorescent protein in layer 5 pyramidal neurons of the cortex. Four week old mice were either monocularly deprived (MD) of light, wherein a shade cloth was used to cover one eye, or they were subjected to auditory fear conditioning (FC), wherein an unpleasant foot shock was paired with an auditory cue. Dendritic spines were imaged via a thinned cranial window in the visual cortex of MD awake mice and frontal association of awake FC mice, using transcranial two-photon microscopy. Sleep deprivation was induced by gentle handling, either over a four-hour period, or during specific sleep stages: rapid eye movement (REM) sleep and non-REM (NREM) sleep. Electroencephalography and electromyography recordings were taken to confirm that the gentle handling was inducing sleep deprivation, and to determine sleep stage. Imaging was performed over 4 hours after one session of sleep deprivation, and then again after a second session of sleep deprivation.
Next, the authors wanted to determine whether the change in rate of spine elimination was reflected in changes to neuronal activity. They measured the amount of intracellular calcium, indicative or neuronal activity, in the layer 5 pyramidal neurons of either the visual cortex for MD mice or the frontal association cortex for FC mice. Finally, to examine whether dendritic calcium spikes during REM sleep are important for dendritic spine elimination induced by MD or FC, they inhibited neuronal activity with an NMDA receptor antagonist at the beginning of REM sleep.
What did they find?
The authors observed an increase in dendritic spine elimination during a 24 hour imaging period in MD mice relative to mice that were not sleep deprived in the primary visual cortex, in line with previous findings. This is indicative of circuit remodeling during critical periods of development. Conversely, following FC, the authors observed an increased rate of spine elimination and formation in the frontal association cortex of FC mice, suggesting that FC induces rapid changes in dendritic spine remodeling. In the first 4 hours of recording following sleep deprivation, the authors observed no significant effect on spine elimination or formation for the MD group overall, and for REM and NREM sleep investigated separately. However, they did find that following a second round of sleep deprivation, the rate of spine elimination and the rate of neuronal activity (as measured by calcium release) was decreased, with no changes to the rate of spine formation.
Interestingly, sleep deprivation, particularly in the REM phase, decreased the rate of freezing in response to the conditioned auditory cue in FC mice, as well as the rate of spine elimination and neural activity of layer 5 pyramidal neurons in the frontal association cortex. In both groups, the authors found that dendritic calcium spikes were higher during REM sleep. The blockade of these spikes via an NMDA receptor antagonist during REM sleep reduced dendritic spine elimination, indicative of the critical role that calcium spikes during REM sleep play in facilitating experience dependent dendritic spine elimination.
What's the impact?
This study found that REM sleep is critical for experience-dependent synapse elimination and modulation of neuronal activity. The authors present compelling evidence for two different types of experience induced change, either via monocular deprivation or auditory fear conditioning, in two different brain regions. This further strengthens our understanding of the role that sleep plays in supporting plasticity in the brain during development and in response to learning. Future work may investigate the mechanisms underlying calcium spike-dependent spine elimination.
Zhou et al. REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nature Communications (2020). Access the original scientific publication here.