Human Brain Organoids Can Integrate into the Adult Rat Visual System
Post by Megan McCullough
The takeaway
Human brain organoids, grown from human stem cells, can integrate both structurally and functionally into adult rat brains.
What's the science?
One promising treatment for restoring brain function after an injury is cell transplantation. Human brain organoids, created from pluripotent stem cells, are an avenue of research currently being studied for therapeutic potential. Previous studies have shown that it is feasible for human brain organoids to integrate into rat brain systems, but there has been limited research into the functional integration of these organoids into the networks of injured mammalian brains. This week in Cell Stem Cell, Jgamadze and colleagues transplanted human brain organoids into damaged cavities in the visual cortex of adult rats to examine potential integration.
How did they do it?
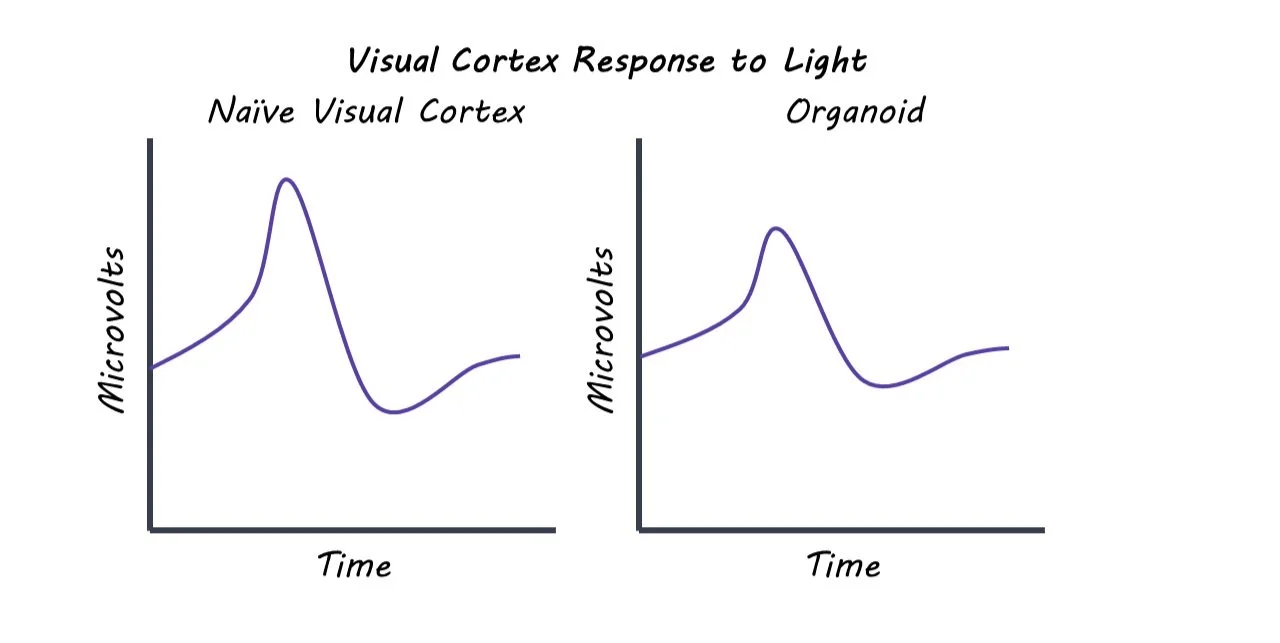
The authors transplanted brain organoids into the visual cortexes of rats. These tissues were generated from human stem cells and expressed the fluorescent marker GFP - a protein that lights up once exposed to ultraviolet light, allowing the authors to identify the organoids once grafted into the rat brains. The organoids had been growing for around 80 days, longer than in previous studies, to allow for maturation and cell differentiation. The authors then conducted a histological (tissue) analysis on the human grafts to confirm the human origin of the cell tissues, examine potential cell maturation, and observe any immune response from the host tissue. This was done to examine how the cell grafts evolved over time. Viral tracers were also injected into the eyes of the rats which allowed the authors to observe the connections between the human cells and rat cells. Next, the authors looked for functional integration of the transplanted organoids. Extracellular recordings were conducted to test for neural activity in the grafted tissue in response to presenting the rats with visual stimuli.
What did they find?
The authors found that in a three-month time period, human brain organoids integrated into the visual system of rat brains. Histological analysis showed that although there was inflammation at the graft site, there was only a mild immune response from the host tissue. This suggests that cell transplant can be a viable option into mammalian brains. This analysis also showed that the human cell tissue continued to differentiate and mature once transplanted. In addition to structural integration, the transplanted organoids also showed functional integration. The grafted human neurons received inputs from the visual system, forming connections via synapses with the host neurons. The brain organoids demonstrated both spontaneous and evoked neural activity, providing further evidence of functional integration. When the rats were exposed to flashing lights, the organoid neurons responded to the stimulation in similar way that the host cells responded.
What's the impact?
This study found that human brain organoids can both structurally and functionally integrate with the visual system of rats. The human neurons formed connections with the rat neurons and displayed electrical activity in response to visual stimulation. This research shows the possibility of using lab-grown neural tissue to reconstruct brain circuitry after brain injury or stroke.